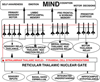
HOW THE BRAIN WORKS
INTRODUCTION "How the brain works" points-out components of nervous system organization and functions that control behaviors. The nervous system is sculpted within a tube of glial astrocytes that form a skeletal-core for neurons to build circuits. This glial tube is made-up of radially directed glial cells that span the tube from a central canal to a pial surface. During development, the diameter of the tube allows full glial extension from the tube surface to the central canal but this relationship is lost in the adult NS because of the massive increase in size. The tube architecture is the base for the spinal cord and brain stem and then continues into the cranial vault forming the brain. The space between astrocytes is where neuronal circuits form by holding the space open between astrocytes allowing excitable cellular elements to grow long axons to make synaptic connections between spinal cord, brain stem, basal ganglia and cerebral cortex. The neurotube part constitutes the central nervous system (CNS) while peripheral neurons group into ganglia with their axons forming peripheral nerves to body organs (PNS). The neuronal basis of primitive behaviors is through sensory nerves that make connections to motor organs that contain muscles or glands. These peripheral connections form local reflexes through the spinal cord and brain stem and establish the most primitive behaviors that arise from the spinal cord and the brainstem. Complex circuitry of neurons form automatic patterned movements of behaviors that arise from basal ganglia in conjunction with the cerebellum and cerebral cortex. While many behaviors are sensory-motor reflexes, volitional behaviors are initiated by the conscious MIND (see Understanding the Mind, mindoverbrain). The MIND emerges from brain function with activation largely through sensory inputs and memory. The sensory inputs are gated into the cerebral cortex by synchronization of thalamic relay nuclei that temporally integrate motor with sensory inputs, memory, emotion, cognition and speech content.Memory systems capture emotions incorporating them into cognition yielding an expression of intellect and perception. Together with decision-making, the conscious MIND emerges to initiate willful acts and to formulate speech content. This presentation offers access to free teaching materials on how the brain works via neuronal circuits for controlling behaviors and generation of the MIND. Reference MATERIALS provide detailed descriptions of how neurons function to generate circuitry yielding behaviors and the conscious MIND. CLICK ON RIGHT-SIDE IMAGES TO ENLARGE AND VIEW DESCRIPTIONS. See also mindoverbrain.com Dean E. Hillman, PhD |
 | |
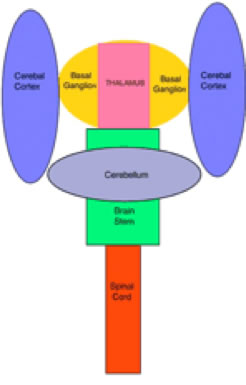
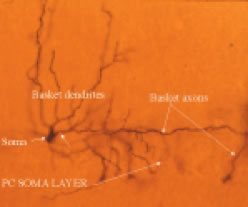
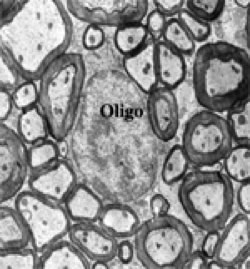
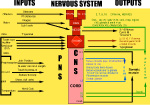
ORGANIZATION OF THE NERVOUS SYSTEM The Central Nervous System (CNS) includes the brain and spinal cord. The CNS is sculpted as a neurotube that forms a continuous space for neurons to migrate and make distant connections in the formation of neuronal circuitry. A peripheral nervous system (PNS) connects body parts to the CNS through nerves. Together, the PNS and CNS compose numerous neuronal circuits that yield a broad array of functional organizations controlling body and mind. Neurons, astrocytes, and oligodendroglia are the principal cells that form the Central Nervous System. Neurons are polarized, electrically excitable, cells. They are characterized as having a receptor pole and an effector pole. The receptor pole is most often the neuronal cell body (soma) and multiple emerging dendrites, while the effector pole is a single axon. The receptor pole acts as the input region on which each neuron receives communications of other neurons.The effector pole (axons) acts to conduct signals to target neurons at some distance from the neuronal cell body site. Axons end in synapses on dendrites and somata of other neurons. These axons carry and liberating transmitters at distant targets. Most characteristic of neurons are dendrites that spread within target sites of axons. Dendrites branch to reach out to maximize input connections of axonal terminals or else limit specific types of inputs. Dendrites polarize neurons by having specific receptors. The two regions of neurons are separated by the conduction pathway (axon) that connects specific neurons with each other. Numbers of neurons participate to form circuits. Astrocytes organize radially into a cellular neurotube that extends for most of the length of the spinal column and forms the brain in the cranium. These glial cells are arranged radially from a central canal and ventricles to the outer surface of the CNS where they form the pia. Dura is a tough connective tissue that lines the bone and has inward folds between the hemispheres and cerebellum that support these brain parts from each other. Early in development, the astrocyte nucleus can be located at any position in the wall between the two surfaces or between a blood vessel and neurons. Because the neurotube wall thickens so much, astrocytes can not span such lengths and thus become detached from one or both ends so that most become isolated in the neurotube wall ending-up as a cellular connection between blood vessels and neurons. Astrocytes link neurons to the vasculature for supplying neurons with oxygenation and metabolic components. Most important, astrocytes are keepers of open-space within the neurotube. A continuous intercellular space between astrocytes is maintained for accommodating neuronal cell bodies forming groups of neurons called nuclei. The continuous open space between astrocytes is especially important for extension of axons that grow for distances in bundles forming pathways. These bundles connect groups of neurons of the spinal cord, brain stem, basal ganglia and cerebral cortex. Connecting axonal bundles allow formation of widely diverse circuitry that controls brain function, expresses behavior and forms the conscious mind. Oligodendroglia isolate axons separating them as individual connectors. The cell membrane of oligo's forms multiple wrapping's around neuronal axonal projections. These wrappings of cell membrane form compacted layers that act to insulate neuronal axons from cross-talk, while controlling the speed of axonal signal conduction and for sustaining uni-directional progression of the action potentials along the axon.
|
;
| |
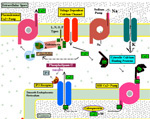
NEURONS IN BRAIN FUNCTION Neurons are ion charged cyosolic bodies yielding an electron sink that is specific to excitable cells. Intra-neuronal space (cytosolic space) and inter-neuronal space (tissue space) are two functional compartments in the nervous system. These two spaces are characterized by having different ionic and electrotonic concentrations across a limiting-membrane (the plasma membrane) that encloses the neuron. This plasma membrane contains pumps and acts as a barrier housing ion and electron pores that open and close for signaling either via ions or electrons. Communication between neurons occurs via ions as action potentials and transmitter release. Electron concentration levels are changed along axons and at synapses of axons onto dendrites or neuron somata. These electron potential changes are conducted between neurons as signals that are carried by axons. Each neuron is constantly trying to maintain a resting potential by pumping out sodium and calcium from each neuronal cytosol. Meanwhile potassium enters neurons creating an electron sink in the cytosol. | ||
|
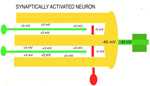
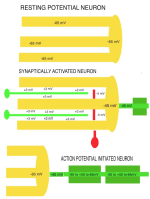
RESTING POTENTIAL Although neurons are never at rest, there is an average negative voltage level in the cytosol and axoplasm that serves as the background on which all functional voltages reside. This negative cytosolic voltage level of -65 mV is generated by potassium entering the cytosolwhen sodium is pumped out. This voltage is sustained by low ionic concentration of Na and Ca in the cytosol while potassium passively enters the cytosol to make-up for positive charges of Na and Ca that are in low concetration there. The cytosolic-potential is produced by positive ions of potassium flowing into neurons. Potassium ion entry into the aqueous cytosol produces an electron sink. Potassium , sodium and calcium ions in water highly ionize releasing ions and electrons into the aqueous medium. The cytosol acts as a sink that attracts electrons to move into the neuronal cytosol from the tissue space when synaptic activity opens electron pores for electrons. Each synapse raises the voltage level by 3 mV at each site. The reason for the negative neuronal cytoplasmic voltage is not oxidation or reduction for production of the electro-motive-force (EMF) as is found in batteries, but rather to the mere fact that aqueous-ionized potassium has 2X the mass as sodium plus it has one more electron shell. This could explain the electron source while the differential concentration of potassium versus sodium produces the -65 mV electron sink in the cytosol. Cytosolic pumping of sodium and calcium ions requires energy from ATP. A lack of oxygen for as little as 4 minutes interrupts ATP production and renders the individual unconscious due to calcium and sodium build-up inside of neurons. Longer anoxic periods produce permanent damage to neurons because excess of calcium inside of the neuron is known to break-down microtubules in dendrites. The integrity of neuronal cytoskeleton is formed by polymerization of tubulin monomers during development and can not be replicated easily in mature neurons. Sustaining of coma for long periods after anoxic events may be helpful in re-polymerizing microtubules in dendrites back to a level that synaptic connections can be supported in number required for circuit integrity. |
|
|
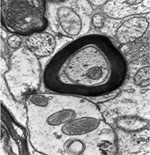
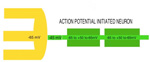
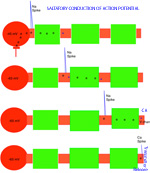
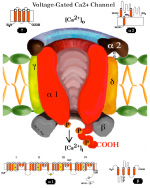
SYNAPTIC POTENTIALS Synaptic junctions occurring between neuronal plasma membranes are typically marked by molecules that sustain intercellular contacts and participates in signal processing. The presynaptic membrane specialization is typically shrouded by synaptic vesicles containing transmitter. Connnectivity is formed by synapses that are characterized by a neuronal transmitter type and one or more specific receptor targets. The source of synapse differences are in transmitter chemistry and their targets as receptor types. Receptor types and their linkage to target neurons are the most variable factors of synaptic signaling. Neuronal signaling occurs on the soma and dendritic poles as excitation and/or inhibition. Excitatory synapses are principally represented by acetylcholine, glutamate or aspartate transmitter. These transmitters act temporally by opening pores on the postsynaptic side of target neurons, muscle or gland secretory cells before transmitter inactivation. Glutamate transmitter is wide spread as the temporally operated transmitter of the CNS. Acetylcholine classically operates at the neuromuscular junction as well as at neuronal synapses in the PNS and CNS. The type of connections forming circuits is determined by the type of transmitter and the type of receptor. Temporal excitatory connections are mostly glutamate or aspartate transmitters and have matched receptors. Glutamate transmitters bind receptors on the surface of target neurons to open channels on the postsynaptic site opposed to the receptor. There are quite diverse populations of glutamate receptors as based on multiple components of target-neuron receptor populations. Glutamate receptors molecules are represented in numerous protein subunits (NR1, GluR I, GluR II, GluR III, GluR IV, etc.) that join to form specific properties of glutamate receptors. A rapid break-down of glutamate transmitter to glutamine by astrocytes recycles glutamate back to neuronal transmtter. The rapid uptake of glutamate with break-down to glutamine is by astrocyte projections that cap and surround synaptic sites. Inhibitory synapses transmit via GABA or glycine. Inhibitory sites have GABA or else glycine as transmitters. These inhibitory connections are represented by targeting chloride channels that open postsynatically with GABA or glycine. As a result, chloride ions pull-in more potassium to further lower the cytosolic mV potential from -65 to as much as -90 mV. The GABA receptors are GABA A or else GABA B. GABA A acts as a temporal counter to glutamate excitability by pulling-in more potassium. GABA B receptors are modulatory and have much longer activity durations by limiting GABA transmitter release and inactivation. Chloride ions move into the target neuronal cytosol due to opening of chloride channel that open as receptor activated pores. Correspondingly, the negativity of the cytosol goes from -65 mV to as much as -90 mV. Activation of inhibition occur by even more potassium ions moving into into the cytosol in order to increase negativity to a -90 mV resting potentials. This results in further increases in the deficiency of electrons in the cytosol. The result is that more excitatory synapses are required to depolarize target neurons to trigger an action potential. Thus, the neuron has reduced excitatory sensitivity. Acetylcholine is a major excitatory transmitter in CNS and PNS neurons as well as muscles that targets nicotinic cholinergic receptors. Acetylcholine appears to be also modulatory with long duration targeting of smooth muscle, glands and cardiac muscle having muscarinic receptors. Pairing of transmitter and receptor types either activate (excite) or inactivate (inhibit) neuronal targets. Excitatory synapses appear to be associated with electron size channels rather than ion sized pores as is suggested by the small 3 mV level change at each synapse. Ionic postsynaptic sites crossing neuronal plasma membranes would produce over +50 mV changes in cytosolic voltage, swamping synapses at only 3 mV. This suggests that the presynaptic mechanism is very different than the postsynaptic component. Postsynaptic Channels- Ionic or electrotonic? A long held dictum has been that ions move into target neurons at synaptic sites. However, the possibilities of ions generating only 3 mV synaptic potentials on target dendrites or somata seems entirely unlikely since ion depolarizations produce over +50 mV spikes when they trigger sodium channels in axoplasmic membrane. Chemical transmitters are liberated from axon terminals by axonal action potentials. Transmitters open pores at the postsynaptic membrane of targeted postsynaptic neurons. Most postsynaptic channel descriptions presume sodium channels as ion pores produce large trans membrane potentials in positive voltages. This author proposes that synapses have a chemical transmitter component and an electrogenic one. Having an electron size pore at postsynaptic sites would be consistent with 3 mV synaptic potentials and their summation for signaling via the cytosolic resting potentials. The postsynaptic membrane is lined with receptor proteins that are sensitive to specific transmitter proteins. The receptor proteins are linked to membrane channels that open and let in electrons in amount that change the cytosolic voltage by + 3 mV. Previously, investigators thought these to be ions because synapses were deemed to be all chemical. However, the identification of connexin proteins and GAP junctions offers means of finding the source for an electron size pore coupling postsynaptic receptor sites at temporal and modulatory synapses. The chemical component is the chemical transmitter that is released at presynaptic sites while an electron component is represented by postsynaptic receptor opening of an electron permeable pore at the targeted synaptic contact. The recent demonstration of electrotonic gap junction-proteins, called connexins, occurs as electrotonic channel pores between neurons by aligning two proteins across the two neuronal plasma membranes forming a pore through which electrons easily pass between opposed cytosols as gap junctions. TEMPORAL VERSUS MODULATORY SYNAPSES There are 4 major categories of synapses: Temporal, Modulatory, Metabotrpic and Gap junctions. The majority of synapses are chemical transmitter based (the exception is gap junction) and require voltage-gated calcium to releases transmitter at axon terminals. Synaptic sites include an axonal terminal with transmitter production, vesicle formation, vescicular transmitter release and a postsynaptic target neuron with a receptor site for signal coupling. Each temporal synapse produces a 3 mVolt depolarization in the soma or dendrites and these sum temporally in the cytosol of the targeted neuron. The electrons equilibrate throughout the entire soma and dendrites at near light speed but are stopped by the narrow initial segment and the cylindrical shaped axon. The summation of synaptic potentials occur at temporal excited sites occurring at sites within 3 msec alters the neuronal cytosolic voltage reaching levels that activate action potential in the initiial segment of the axon. Action potentials use a combination of sodium ions and electrotonic signaling to send signals down axons to synapses forming circuits. The fidelity of brain function is in timing within Neuronal Circuitry. Neurons of various types are linked temporally through axonal connections to dendrites and somata through synapses. Transmitters of temporal pathways are either excitatory or inhibitory and are active for only 3 msec or less. This restriction in activity-time is due to de-activation of the transmitter forming a 3 millisecond window for excitatory synapse activation. The temporal limit of synapses defines a period of relevancy where summing of connections with each other occurs with a 3 mV increase in the neuronal cytosolic potential over a 3 msec interval. This time frame is the period for summation of numbers of synaptic sites as excitatory or inhibitory. The sum of individual neuronal afferents and the temporal frequency of action potential responses encodes the message signals of axonal outputs to target neurons. Each synaptic depolarization raises the neuronal potential by 3 mV from a present negative to a less negative value. Synaptic voltages of 3 mV last for only 3 msec. Thus, the number of active synapses that summate in time producing a temporal response via an action potential is determined by connections in neuronal circuitry. Electrons having entered at synapses move freely through-out the cytosol of dendrites and soma and out to the initial segment where they are stopped by the small diameter of the initial segment and the cylinder shape of the axon. Signaling within and between neurons utilizes the -65 mV electron potential of the cytosol as compared to extracellular tissue space. Synaptic voltage-shifts occurring within the 3 msec summation period equilibrate voltage across the soma/dendritic cytosol at near light speed. Free electrons from ionization are recruited from the tissue fluid space and enter the cytosol through synaptic receptor opened electron pores. Active synapses that depolarize the cytosol from -65 mV up to -45 mV initiate the axonal action potential (AP) in the initial segment by opening sodium channels. An action potential is propagated by electrons being spread to subsequent successively adjacent nodes. ACTION POTENTIALS Action potentials (AP) communicate the summated soma/dendritic excitatory and inhibitory synaptic signals to the next neuronal components of circuits. The minus 65 mV potential in the soma/dendritic compartment of the neuron is not strong enough on it's own, for its signal, to reach distant neurons. Therefore, evolution has developed the action potential as a sodium entry spike that is replicated up axons as saltatory discharges for as much as 2 meters in the human species and many more meters in large species. The strongest signals in neurons are produced by ions of calcium and sodium. However, ions are comparatively large and can not move very far in the short time requirement. Pumping of these two ions from the neuronal cytosol into the intercellular tissue causes potassium to equilibrate the number of positive ion charges between the inside and outside of neurons. Potassium is the positive ion that balances ions because they are most permeable to the neuronal membrane and are not affected by sodium and calcium pumps. The sodium signal must be propagated from node to node by electron voltage activation of sodium channels generating an electron field producing a nodal sodium spike with their electrons spreading in the axoplasm to the next node where sodium channels are again opened to form a spike. Ions do not move significantly from any one node region, so that advancement down the axon is by electrons spreading in the axoplasm from the spiked node to the next sodium voltage sensitive node on the axon where sodium channels open and sodium ions rush in. Thus, the sodium axonic signal is led by an electrotonic field spreading to the subsequent node of Ranvier moving in a saltatory progression from node to node. In axons, electrotonic spread is stopped by the cylinder-shaped axon diameter and myelin inter-node length. Electrons can not spread beyond one or two myelin nodes because of limits on ion spread in the cylinder shaped axon. As a result, conduction speed is limited from less than a meter a second to about 100 meters/sec in mammals. This speed control optimizes synchrony between inputs. The signaling for axon potentials requires an initialization phase that parlays a small reduction in cytosolic voltage potential of from -65 mV up to -45 millivolts. This 10 to 20 mV depolarization triggers opening of sodium channels in the initial segment or at the next node of Ranvier of the axon. This produces an ionic spike in that local region of the axon. The electron field around the spike spread up the axoplasm to the next node where sodium channels open to produce a spike at the next node of Ranvier. Electrons emitted from around the sodium spike at each node spreads freely into widening space of the dendro-plasm but are not free to flow down the axo-plasm for more than one or two nodal segment lengths. The restriction difference is that electrons spread within the axoplasm by the volume being a cylindrical shape. The constant cross-sectional diameter of the axon does not allow ions to flow down axons for any significant distance while electrons flow down the axoplasm for at least one node. The rise of the sodium voltage spike lasts only 0.1- 0.3 msec during which time there is an influx of sodium through open sodium channels. This is followed immediately by a slightly slower eflux of sodium. This nodal spike of 1 to 3 ms, in sodium is accompanied by a slightly delayed eflux of potassium ions from the initial segment axoplasm or at a node and an immediate influx of potassium to re-balance the positive charges of sodium going out. The limit of axonal spike potentials to 1 msec also limits the firing rate of neurons to 1000/sec. The delayed potassium spike is essential in order to prervent the AP from reversing directions, once initiated, as well as so that axon diameter and internodal length can control the speed of conduction. The control of speed is means to ensure arrival of action potentials at optimum times for maximizing summation of synaptic events at targets. An electron field is generated around the node accompaning the ionic spike. While sodium can not move very far in this short time, electrons spread up the axon to the next node of the axon where electrons open sodium channels at that node. The opened sodium channels produce a local spike of 50 plus millivolts and let in electrons that open ion channels at the next node. Progression of the electron spread to the subsequent nodes produce an action potential along the axon in a saltatory manner. Thus, electrons trigger opening of sodium channels at the next near node producing a local sodium spike while the electrons around the spike spread to open sodium channels at the next local adjacent node to open sodium channels. A lengthy after-hyperpolarization prevents direction reversal. The result is saltatory progression of the action potential (AP). Potassium eflux accompanies the sodium influx at nodes but with a slight delay (see figure). Thus, the depolarization by the influx-eflux of the sodium spike is followed almost immediately by a hyperpolarization due to a delayed potassium eflux-influx that is regulated by potassium channel opening. The latter produces spike characteristic differentials seen in somatic muscle, cardiac muscle and CNS axons. Such relationships produce timing for preventing AP direction reversal, AP speed control, summation characteristics and muscle contraction durations. The advance of the axonal signals by electrotonic spread is due to the cylindrical shape of axons and the myelin internode distance. These allow the system to control action potential speed and sustain the AP direction. The speed of the AP is held in check by internode length together with axon diameter to the next node of Ranvier. The speed of conduction is controlled so that the AP reaches the target synapses in an optimal frame of time. For example, only when the 3 msec window of AP production at the synapse occurs, can summation occur in the soma/dendritic pole and the AP can become a part of the neuron to neuron signal. TRANSMITTER RELEASE AT AXONAL TERMINATIONS BY VOLTAGE-GATED CALCIUM ION CHANNELS Besides voltage-gated sodium channel triggering of action potentials with saltatory conduction, voltage-gated calcium channels produce release of transmitter from the axonal bouton terminals and onto target neurons. The presynaptic event is an action potential triggered voltage-gated calcium channels that produce an influx of calcium into the bouton. This calcium event brings synaptic vesicles to the presynaptic membrane with joining of their membranes and exocytosis of the vesicle content into the synaptic cleft. The transmitter targets receptors on the postsynaptic membrane of the target neuron.This exocytosis empties vescicular content into the narrow gap between the axon and the neuronal membranes. On the target neuron, the transmitter couples with targeted receptor molecules. The electrotonic signal for activating calcium or sodium -gating is about +10 to +20 mV bringing the axonal voltage to -45 mV. This trigger-voltage for ionic calcium releases calcium into the bouton where it joins synaptic vesicles causing them to open into the synaptic site. The chemical transmitter targets receptors onto postsynaptic membrane of the target neuron soma or dendrites.
|
| |
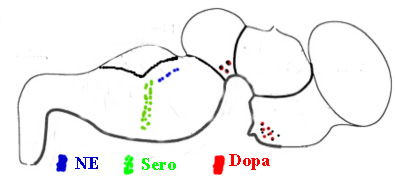
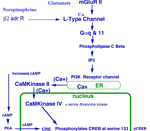
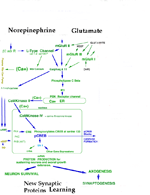
MODULATORY SYNAPSES The second major class of neurons is the modulatory type. These include norepinephrine, serotonin, acetylcholine or dopamine producing. Modulatory transmitters have long duration actions and are released over large target regions. With the exception of acetylcholine, all of these modulatory neurons are located as small groups in the brain stem-(pons, medulla and midbrain). Modulatory transmitters target diverse regions of the spinal cord, brain stem, basal ganglion, and cerebral cortex. Modulatory axons are from local clusters of neurons in the brain stem (examples are substantia nigra for dopamine, locus cueruleus for norepinphrine and raphe nuclei for serotonin). Each of these neurons project axonal terminals to broad areas of the cerebellum and upper brain. Adrenalin produced from the adrenal gland spillover from the blood to the brain where some adrenalin is thought to be produced from adrenalin neurons in the brain. These modulatory transmitter neurons are not dependent on millisecond timing of transmitter release from synapses as found in temporal pathway systems. Axons of the three main modulatory producing neurons send ascending pathways to basal ganglia, amygdala and cerebral cortex. The transmitter is released into free space near receptor targets, rather than, being released specifically into synaptic sites for temporal signaling. The transmitter passes from the soma, down the axon and into vesicle filled boutons and is released through the narrow inter-cellular space to reach receptors. Nevertheless, the receptors of specific temporal pathway synapses co-exist with modulatory types of transmitters. The modulatory systems control up or down sensitivity of temporal synapses by adjusting the resting potential that affects the number of synapses that are required to activate action potentials. The specificity of the modulatory systems is in the receptor type and the precise location of the axonal terminal in relationship to their targeted temporal-synapses that they modulate. These connections are highly associated with behaviors through regulation of motor activity. The slow activation and break-down of modulatory transmitters allow them to affect behaviors that are being processed by temporal synaptic connections. Modulation affects behavior such as aggression versus friendliness or sharing versus expression of power and control. Norepinephrine generally increases actions and counteracts serotonin. Serotonin shows relationships to sedation and sleep. Dopamine modulates basal ganglial activity and is lost in Parkinsons. Adrenergic axons target alpha and beta receptors of which each has types 1 and 2 receptors. These 4 receptor types occur both peripherally and centrally. Furthermore, the receptor can be presynaptic or postsynaptic targeting boutons of axonal pathways. Serotonergic transmitters respond to four receptor types (S1, S2, S3, S4). These receptors are located on pre- or post-synaptic boutons of temporal pathway connections but modulate levels of activity of temporal pathways by regulation lasting for over seconds rather than milliseconds. The reason for the difference appears to be due to the rate of transmitter breakdown or reuptake. Dopamine neurons are highly localized in the midbrain while their axonic targets project most specifically to the striatum and frontal cortex. There are multiple types of target receptors for each modulatory transmitter. For example, dopamine receptors are most prominent in basal ganglia and cerebral cortex targeting four types of dopamine receptors: D1, D2, D3, D4. The actions differ if the receptor is presynaptic or postsynaptic. Dopamine axonal projections target the basal ganglia, hypothalamus and frontal cortex. In the hypothalamus, dopamine is produced in the medial eminence and travels down the neural pituitary stalk to inhibit milk production hormones in the neuro /adenohypophysis organs. Thus blockage of the stalk causes milk production in both males and females when the inhibition pathway is blocked in the hypophysial stalk. Acetylcholine has specific temporal pathways to somatic muscle and to autonomic motor neurons of the PNS, but in addition has a population of CNS, neurons that are modulatory and are dispersed across the CNS. Acetycholine up-regulates motor activity especially in basal ganglia (striatum) to produce directional circling when applied to one side of the brain. ( See Bradford 1986). In addition, there are neurons yielding Neurohormones. The regulatory sensitivity of temporal pathways are further determined by modulator neurohormones and their receptors. The modulatory receptors act faster than neurohormones in that most transport the hormone is through the blood stream. Neurohormones are peptides that are produced by central or peripheral neurons. There are a number of peptides that have specific receptor initiated activities that target localized brain regions. Notably a long list of such peptides include substance P, enkephalin,oxytocin, beta endorphins etc.. Each hormone acts much more specifically than the modulator types as the receptors are highly localized, METABOTROPIC SYNAPSES A third category of synapse is metabotropic type that signals gene expressions. These are glutamate metabotropic synapses that target receptors linking activation of intracellular protein production through signaling gene expression. This glutamate receptor signals G-protein inside of the neuronal cytosol. G-protein alpha q and 11 operate a system forming IP3 via the phospholipase C beta system. This system in turn releases calcium from the endoplasmic reticulum store into the cytosol. This cytosolic burst of calcium signals gene expression by activating IP3 release. Metabotropic glutamate targets IP3 gene expression signaling CaMKinase II in the cytosol. Cytosolic calcium targets CaMKinase II. The CaMK II signals across the nuclear membrane releasing intranuclear calcium that signals nuclear CaMKinase IV to phosphorylate CREB and signal gene expression. Such protein production maybe the source of synaptic proteins that are required for learning. Electrotonic Synaptic Junctions (gap junctions) ELECTROTONIC SYNAPSES The fourth category of synaptic type is electrotonic junctions. An example is the inferior olive that forms climbing fiber connection to Purkinje cells. The plasma membrane of adjacent inferior olive neurons contact with each other and form membrane gap junctions between neurons. Special proteins that are called connexins produce six inter-membrane complexes. The proteins align directly across membranes of contacting neurons forming a pore in the center of the traversing connexin protein mass. The pore size is too small for ions to pass but electrons immediately equilibrate between neurons sharing these gap junctions. The effect is that all of the connected neurons fire action potentials at the same time. Such gap junctions maximize the effectiveness of neuronal groups in unison by producing synchrony between neurons such as climbing fiber axons. The massive release of antagonist muscle groups during ballistic movements (see below) requires synchronous muscle tone by the group. The massive activation of the antagonist group of Purkinje cells inhibit the deep cerebellar pathway in their projection to relay dorsal thalamus motor nuclei. Their role is to produce ballistic movements by synchronized release of antagonist muscles via Purkinje cell action. This antagonist release allows massive ballistic contraction by the agonist muscle.
|
| |
NEURONAL CIRCUITRY STRUCTURAL-FUNCTIONAL ORGANIZATION FORMING NEURONAL CIRCUITS. The primary organization of circuits is based on temporally-defined excitatory and inhibitory synapses that form precisely timed connections as pathways between brain centers. These are 3 msec duration neuronal signals that sum with other events in this time frame to trigger axonal potential propagation's to distant other neurons. Modulatory circuits are brain stem transmitter neuronal systems that by-enlarge have ascending connections to basal ganglia and cerebral cortex. They modulate temporal excitability. The third type is metabotropic activating G-protein for turning on genes through IP3 - Cam Kinase systems. The principal circuits of the nervous system are those that express behavior. Neurons are interconnected into circuits that form brain functions for behaviors. At the base of all behaviors is motor activity. Sensory input must either precede motor action or else the actions are programmed as patterns of movement. Some circuits are prewired and others are programmed by trial and error or consciously improved by specified conscious intention. Circuits are formed of neuronal organizations, as based on combinations of neuronal types, that are defined by their transmitters, axonal targets, and receptor types. These principal factors yield circuits controlling sensory and motor systems, for homeostasis and emergence of the MIND. Circuits are quite specific for each functional system,whether sensory, motor, integrative or driving of the MIND. The critical features governing function are receptor organ structure and effector terminations onto muscle or glands. Temporal sensitivity of synaptic messaging stands at the base of behavioral connectivity. On top of this sequence of timing are up and down regulators of synaptic sensitivity by modulator transmitters. Combinations of features of neurons serve to promulgate the functional role of circuitry: 1) Neuronal dendrites broaden the field of recepotor targets, 2) Soma and dendritic cytosol serve as the final integration site of synaptic inputs, 3) Initial axonal segment initiates action potentials, 4) Voltage activated sodium channels form a field of electrons that activate the subsequent sodium spike at te next axonal node that forms a gap in myelin, 5) Control of conduction speed occurs by axon diameter and internodal myelin segment length, 6) Axonal arbors widen the effector target site, 7) Action potential frequency carries neuron to neuron messages 8) Presynaptic boutons produce and accumulate transmitter in vesicles, 9) Presynaptic bouton depolarization signals transmitter release via voltage-gated calcium channels, 10) Synapses of temporal pathways integrate excitatory and inhibitory transmitter connections for action potential activation. 11) Modulatory transmitters, dopamine, norepinephrine, acetylcholine and serotonin modulate activity in temporal pathway synapses through persistent receptors that de gradate transmitter very slowly. 12) Gap junctions group neurons into synchronously-active units. 13) Voltage-gated ion channels act as a temporal factor in signaling transmitter release between neurons. 14) Temporal summation of action potentials sustain muscle contraction and gland secretion, maintain sensory signal durations and control speed of axonal conduction. Together these features yield circuit operations that express an array of behaviors. Specialized neuronal circuits manage homeostasis, form sensory input, initiate consciousness, gather information for memory and cognition and set-up automatic and volitional motor controls expressing behavior. The basis of consciousness leads to generation of memory, emotion, understanding, decisions-making and speech content. The most significant circuit of the CNS is for consciousness. Alerting senses such as pain, sound and joint movement are the most effective means of awakening the MIND to consciousness. Sensory systems parcel sensory status into specific sensory regions of cerebral cortices by special gating through the dorsal thalamus. Sensory inputs are gated as short bursts into primary and secondary cerebral cortices every 20 to 30 milliseconds by the dorsal thalamus. By maintaining a repetitive sensory stream into pyramidal neurons, the sensory cortices refresh at a rate that produces a sustained activity in pyramidal cells creating a CONSCIOUS STATE of sensory awareness (See source of Mind). The sequence of neuronal events forming a circuit is contained in reverberating loops that consist of cytosol/soma depolarization to -45 mV. This voltage level activates the initial axonic segment to open voltage gated sodium channels. The voltage level change produces an electron field that spreads to subsequent nodes of Ranvier. This triggers a sequence of propagated high voltage spikes at subsequent nodes of the axon. Upon reaching the axonal presynaptic termination, voltage-gated calcium channels activate transmitter releases from synaptic vesicles. Each synapse participates in summation in the cytosol over a 3 msec duration whether excitatory or inhibitory. Adding to these temporal systems are modulation synapses that up or down regulate the excitability of these temporally defined circuits. The latter act over longer time frames with less specific connectivity. The transmitters and receptors of the modulatory systems spread nonspecifically over some brain regions, others are in limited locations and act specifically. Neurohormones are distributed via the vascular system. The temporal circuits are further enhanced by gap junctions that sync numbers of neurons to the same time frame. Furthermore, voltage-gated calcium channels in dendrites produce barrages of Purkinje cells that signal ballistic actions. Other temporal connections activate genes for adaptation and learning. BEHAVIORS ARE BASED ON MOTOR ACTIONS INITIATED IN SENSORY CENTERS Spinal and cranial nerve reflexes are the simplest motor actions and appear to lack cerebellar control. Reflexes generate simple behaviors that are produced by sensory inputs that loop through the CNS making direct connections with a limited number of neurons. Reflexes give rise to low-level CNS and PNS behaviors that emerge from sensory inputs directly to motor output through sensory and motor nerves of the spinal cord or brain stem. Reflexes are expressed through skeletal musculature as well as sympathetic and parasympathetic systems controlling glands, smooth muscle and the heart. The main characteristic of basic reflexes is that they operate without cerebellar control and usually produce a single sweeping action without concurrent production of muscle tone until the end of the movement. Two main examples are extensor stretch receptor reflexes and flexor reflexes. Stretch receptors have a single synapse between sensory neurons and motor neurons. Although the motor neuron can be modulated by descending connections, the stretching of the muscle tendon of a relaxed extensor muscle produces a single extensor muscle contraction that is seen as a jerk. An example is the knee jerk. Dual synapses in the cord form the withdrawal or flexion reflex. An example is the response to a hot surface where sensory control is mediated through a sensory afferent, a single interneuron and a motor neuron form a two synapse reflex arch. The flexor muscle group withdraws the limb as rapid as the two synaptic sites and afferent and efferent axons allow. Many other reflexes are associated with the autonomic nervous system. These are represented in sensory system of the visceral organs as drivers of visceral motor control of smooth muscle, cardiac muscle and gland secetions. |
|
|
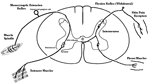
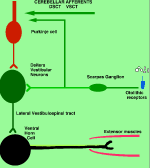
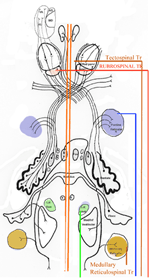
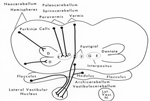
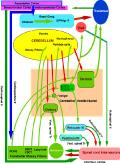
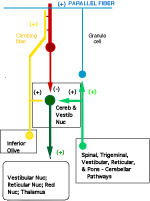
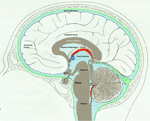
Brain Stem Automatic Patterned Behaviors Humanoid advancement in behavioral pattern control operates through brain stem motor centers, basal ganglia, thalamus, cerebral cortex and cerebellum. Motor expression requires a massive amount of sensory input in combination with inputs from the cerebral cortex. The bulk of nervous system mass, having to do with complex behaviors, involves brain stem, cerebellum, basal ganglial and cerebral cortex control. Behaviors arise from multiple levels of motor control of skeletal muscle through complex sensory-motor actions involving the cerebellum of which we are mostly unaware. Complex motor responses form our behaviors and stand in hierarchical order of actions from brain stem to include the basal ganglia, cerebellum and upper brain. Brain stem neuronal systems are the foundation of automatic patterned movements that emerge as our behaviors. We are generally unaware of the patterns of movements that form our behavioral-image. Each individual has their own characteristic pattern of movement stemming from brain stem centers (vestibular, medullary, and pontine reticular motor systems) with the cerebellum producing muscle tone via agonist and antagonist co-contractions over these motor centers. These operate unconsciously to produce automatic patterned movements. Complex patterns of movement are expressed in automatic motor patterns through genetic wiring of brain stem motor nuclei with control by the sensory systems. Clearly, brain stem centers and related parts of the cerebellum of ungulates are prewired for patterned movements in utero and are activated for standing and running at birth. In the human, the vestibular system must be trained together with the cerebellum in order to produce standing and stepping. Each brain stem motor center has descending pathways to spinal cord motor centers and brain stem nuclei. Those of the vestibular sensory systems have descending pathways to the spinal cord. These are medial longitudinal fasciculus (MLF) and lateral vestibulospinal tract for the otolithic vestibular system. This latersl vestibular system manages the otolithic sensory input, while the canal sensory vestibular system controls eye movements. Standing is a principal behavior of survival and requires the vestibular system to function as a 3-space sensory system. The vestibular outputs are Deiters cells of vestibular nuclei that send excitation, directly, to motor neurons via the ipsilateral lateral vestibulospinal tract driving extensor muscles for standing. (See figure description). Bipedal erect standing is produced by the combination of cerebellar tone across the hip joint through sensory input to the cerebellar cortex through mossy fibers from vestibular otolithic system. Modulation of the vestibular motor system is by cerebellar Purkinje cells that balance the vestibular motor activity through direct inhibition of Deiters cells as the individual stands. The agonist and antagonist co-contractions of musculature are controlled by reciprocal inhibition of agonist versus antagonist muscle groups via Purkinje cell inhibition of the deep cerebellar pathway. The result is smooth movement for standing (via mossy fibers) or tone as a prelude to ballistic movements via climbing fibers signaling of the Purkinje cell inhibition of antagonist muscles. The next most basic patterned movements are stepping or crawling as prime motor functions. The medullary brain stem generates automatic movements through the medullary reticular and pontine reticular nuclei. In the lower brain stem are bilateral medullary motor nuclei with descending crossed and uncross descending medullary reticular pathways to spinal cord facillatory motor neurons. Bilateral innervation of the lower extremity can be synchronized by these dual pathways to produce stepping. The medullary reticular pathway is the automatic stepping center described by Sherrington. MM medullary reticular nuclei send bilateral decsending pathways to ventral horns where they synapse on fascilitory motor neurons that distribute bilateral stepping signals to the correct muscle groups serving stepping behavior. The cerebellum generates the extensor tone during each step. The next higher center (pontine) provides automatic movements for unilateral arm movements. The pontine reticular center generates a unilateral descending pathway to spinal and brain stem motor nuclei. These projections provide automatic discrete movements like writing, speech, smiling and other facial expressions from the pons motor center. The ipsilateral course of this pathway is likely related to unilaterality of actions and the handedness of the upper extremity. The pontine reticular center is unilateral projecting to the same side musculature of the brain stem producing even more elaborate automatic behaviors related to trunk and proximal limb musculature. The red nucleus stands above the other brain stem centers. Located at the midbrain, it has both ascending thalamic connections to the cortex and descending pathways for generating automatic motor control. Crossed descending rubrospinal pathway from red nuclei project to motor nuclei of the cord and brain stem at the ventral horn. This crossed bilateral rubrospinal tract is to the cervical spinal cord motor neurons particularly serving the upper extremity. The descending path projects contralateral to cord facillatory motor nuclei with synapses on interneurons of the ventral horn. The target is to the ventral horn of intermediate proximal musculature of the forearm/hand and foot/leg. This motor pathway produces higher discrete movements than the medullary and pontine nuclei. The ascending limb generates a conscious component of the red nuclei projects to the cerebral cortex via the dorsal thalamic ventral lateral and ventral anterior nuclei. This allows MIND choices or automatic behaviors via motor and premotor cortices. CEREBELLAR ROLE. The cerebellar control targets all automatic motor centers of the brain stem. The purpose is to provide tone to movement during automatic actions.Tone in posture is generated by co-contraction of agonist and antagonist muscles. The deep cerebellar pathways is driven by collaterals of sensory inputs or pons and inferior olive having control from the cerebral cortex. PC's dynamically inhibit the deep cerebellar pathways via two systems: mossy fiber sensing pathway for tone in dynamic movement or else through climbing fiber input to PC's for ballistic movement. Control of tone in muscles is produced by dynamic output of Purkinje cell inhibition on to the deep cerebellar nuclear pathway that targets the dorsal thalamus as a temporal relay to premotor and motor cortices. Tone must be maintained as a dynamic process that occurs during all movements except reflex and ballistic movements. The sensory pathway for tone input modulates standing through mossy fiber inputs of hip joint stretch receptors, vestibular otolithic inputs and skeletal joint receptors. These sense the standing position and feed foreward cerebral inputs adjusting agonist and antagonist muscles, dynamically, during erect standing. Processes for tone are transmitted to the cerebellum from sensory input to the cerebellum, and the cerebral cortex via mossy fibers through the pons. These series of motor control centers are represented as an overlapping continuum of innervation forming an ascending hierarchical order of motor control. The cerebellum adds tone control for all musculatures from proximal to distal groups. This continuum is also represented in the cerebellum where brain stem motor centers receive tone control from midline cerebellum while basal ganglia control cerebral motor regions receiving increasingly lateral connections from the cerebellum. |
||
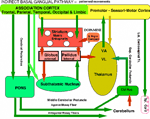
Basal Ganglial - Automatic Patterned Behaviors and Volition The dorsal thalamus connection to the cerebral cortex is the basis of consciousness and MIND determination. Automatic and volition patterned movements are generated through the basal ganglia. Behavioral awareness is realized through basal ganglial projections to the dorsal thalamus and from here to the cerebral cortex. The goal of automatic movement is determined by volitional control by the MIND but are processed, unconsciously, by the basal ganglia/cerebellar system. The basal ganglial pathways control behaviors especially for distal extremities with dexterity being expressed in hands, fingers, feet and facial expression (smiling). The motor components of these automatic behaviors are gained through unconscious conditioning of movement patterns in the upper brain stem, such as the red nucleus, and in basal ganglia/cerebellum. Integration of cerebellar control with basal ganglial output are brought together as cerebellar control by the deep cerebellar pathway with integration via basal ganglia in the dorsal thalamus. This combination of instructions is relayed to cerebral cortex for motor execution where consciousness of movements are realized. Two modes of basal-ganglial-control produce two different types of motor behaviors. One mode is through BG via the subthalamic nuclei, the indirect system, and the other path by-passing the subthalamic nucleus as the direct system. The former path defines smooth movements with dynamic control through the cerebellar cortex. The latter produces ballistic movements that are initiated by MIND determination through basal ganglia and cerebellum . Indirect Basal Ganglial Pathway (Automatic Cerebellar Modulated Behaviors) The indirect basal ganglial pathway is the source of smooth-dynamic-automatic-motor-behaviors. The conscious MIND is mostly unaware of the movement processes in behaviors although movements are initiated for behaviors. This indirect pathway arises from the cerebral cortex with the major projection to the striatum (caudate and putamen). From this source, the striatum connects to the external globus pallidus and then to the subthalamic nucleus. The subthalamic nucleus connects back to the internal globus pallidus nuclei to form a pathway to VL and VA motor thalamus where integration with cerebellar control produces tone during fine movements of hands, feet and face by relaying them to the cerebral cortex and then to behavioral motor muscles. This indirect pathway controls automatic cerebellar-modulation of fine movements of behaviors such as hand /finger movement, foot manipulation, facial expression and speech.The indirect system unconsciously signals behavioral patterns once the behavioral goal is decided. The tone of the involved musculature is modulated by the cerebellar cortex by selecting reciprocal relaxation and contraction of agonist versus antagonist muscles by action of Purkinje cell inhibition to the deep cerebellar nuclear pathway. The PC inhibition relaxes tone through dynamic modulation from mossy fiber input to the cerebellum for relaxation of co-contraction muscles of agonists and antagonists groups. These actions are unconscious to the individual as sensory input from mossy fibers of spinal/brain stem sensory inputs and of the cerebral pontine-cerebellar pattern generation systems occurring unconsciously. The mossy fibers input to the cerebellum forms the deep cerebellar pathway by synapses on granule cell inputs to Purkinje cells. The sensory pathways to the cerebellum are ventral (VSCT) and dorsal spinal cerebellar pathways (DSCT). The cerebral cortical expression of movement patterns are from pons mossy fibers. The agonist and antagonist related cerebellar arrays of Purkinje cells are presumably interlaced along cerebellar folia for agonist/antagonist folia from medial to lateral as granule cell /parallel fiber arrays that input dynamic tone status through inhibition by Purkinje cells. The output follows as Purkinje cell inhibition onto the deep cerebellar pathway with integration in the dorsal thalamus (VA ,VL) with basal ganglial movement pattern generation. The VA and VL integrated output projects to premotor and motor cortices for innervating motor neurons for producing tone during smooth movements.
|
|
|
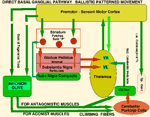
Direct Basal Ganglial Pathway for Volitional Ballistic Behaviors Volitional Behaviors All ballistic movements are volitional. There are two types of volitional movements: intentional discrete movements and ballistic movements. Volitional discrete movements are consciously initiated by MIND. Discrete movements are intention guided by addressing individual movements for precision. Purkinje cell inputs are through mossy fibers modulating muscle tone through agonist/antagonists during discrete movement. Mossy fibers sense tone intensity and signal Purkinje cell inhibitory balance of tone for contractions between agonist and antagonistic muscle groups. The direct basal ganglial pathway is concerned with ballistic movements in volitionally initiated behaviors. The direct pathway projects from the cerebral cortex to the striatum and then directly synapses in the globus pallidus with outputs to the dorsal thalamus (VL and VA). This BG output for ballistic movements is integrated with deep cerebellar output that carries the ballistic release of the antagonistic component of these movements. Together, cerebellar output and direct basal ganglial control merge temporally in the dorsal thalamus from which signals are relayed to the premotor and motor cortices for signaling motor neurons. The cerebellar part of this pathway stabilizes the agonist and antagonist system and then releases the antagonist musculature by Purkinje cell inhibition to the deep cerebellar pathway. The preballistic tone is set-up for movement patterns by the indirect system that stabilizes tone producing co-contractions. The PCs release the antagonist tone by inhibition of deep cerebellar pathway by Purkinje cells producing ballism as the agonist becomes unopposed by an antagonist. Antagonistic muscle release allows forceful contraction of the agonist that makes the ballistic action. This mechanism of ballistic movements are supported by the clinical occurrence of contralateral hemiballism that occurs when a vascular lesion occurs in the subthalamic nucleus and the connection through the basal ganglia has to by-pass the subthalamic nucleus. Cerebellar climbing fibers excite arrays of Purkinje cells which inhibit the deep cerebellar pathway releasing the antagonist component of tone control. Gap junctions between inferior olive neurons ensure massive release of antagonist related Purkinje cells. Climbing fibers singally activate a Purkinje cell. By coupling number of active Purkinje cells through gap junction coupled inferior olive neurons forceful ballistic movement by the agonist contraction can occur. The direct basal ganglial pathway engages the deep cerebellar pathway in the ventral lateral dorsal thalamus (VL) signaling premotor and motor cortices. The hitting of a baseball or golf ball are examples of ballistic behaviors. Yet, these are adaptive behaviors that form movement patterns but can be adjusted by conditioning through trial and error observations and practice. Dendritic Action Potentials: Voltage-Gated Calcium Ion Channels in Ballistic Movements In neurons that have dendrites with voltage-gated calcium channels, action potentials occur in dendrites. Best known are voltage-gated calcium channels in Purkinje cell dendrites. The climbing fibers produce barrages of dendritic spikes on top of massive 20 mV depolarizations of Purkinje cells by voltage-gated calcium channels. This generates a sustained calcium influx into the Purkinje cell dendrites yielding continuous dendritic spikes for as long as 20 msec. This climbing fiber activation produces sustained depolarizations that are needed to produce ballistic actions by that signal Purkinje cell inhibition of deep cerebellar pathways releasing antagonistic muscles. Volition is signaled through the inferior olive with climbing fibers activating Purkinje cells to produce inhibition of antagonist muscles as part of the deep cerebellar path to the ventral lateral dorsal thalamus (VL). Stimulation of Purkinje cells electrically fails to produce obvious movement. This is because cerebellar output reduces muscle tone. Lesions in cerebellar afferent pathways is readily demonstrated by a lack of tone. Volitional behaviors are initiated by the conscious MIND but mostly occur through unconscious brain stem and basal ganglia systems. Conditioned pattern-movements are being continually up graded as we express behaviors. |
||
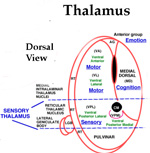
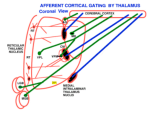
SENSORY SYSTEMS- BASIS OF CONSCIOUSNESS The mark of MIND is consciousness. The common basis for recognizing the existence of the conscious MIND is eyelid opening. During body paralysis, voluntary following-eye-movements are indicative of a high level of consciousness eventhough the entire body may be paralyzed. Sensory inputs to the CNS are the foundation for emergence of consciousness and the activation of the MIND. Sound, pain and physical movement are the most responsive generators of consciousness from sleep and unconscious states. Hyper-stimulation of any one sensory system focuses awareness to that system. Nevertheless, the MIND can direct focus to other sensory inputs even though the signals maybe weak. Specific senses (tactile, deep pressure, vibration) as well as physical joint movement and pressure produce stimuli activating consciousness. Vision has little affect upon awakening the mind from sleep to consciousness. The MIND emanates, primarily, from inputs to pyramidal cell activity within each of four major regions of the cerebral cortex. Inputs for each sensory modality (i.e. vision, audition, body contact, pressure, pain etc) reach sensory sub-regions of cerebral cortices as temporal relays via sensory nuclei of the thalamus. These result in consciousness and self-awareness. In order that sensory inputs reach pyramidal cells with meaning, inputs must be gated into the cortex by thalamic synchronization. Pyramidal cells are initialized by receiving inputs from thalamic intralaminar nuclei through layer one of the 6 layered cerebral cortex. This initialization appears to produce a baseline cytosolic voltage that is followed by sensory input signals. This prevents carryover of signals when changing thoughts. The neurological basis of consciousness is behavior. Consciousness is based in behaviors that are driven by sensory inputs and their associated emotions. At the center of conscious behaviors is standing. The signaling of extensor skeletal muscle contractions produces the ability to stand. This is a complex reflex of the lateral vestibular nucleus that gets input from the otolithic organs and directly innervates extensor motoneurons of the lower extremities. At the same time, the cerebellum PCs inhibit the lateral vestibular nucleus according to the sensory output for standing. Each sensory modality has its own dorsal thalamic target that acts as a relay-gate to respective sensory cortices. Relay nuclei of the dorsal thalamus act as a temporal gate of sensory inputs preventing cortical input during the critical period of pyramidal cell voltage normalization. Gating of sensations to respective sensory cortices occurs as short bursts of sensory data or motor instruction that follows a voltage activation to repolarize pyramidal neurons to a normative voltage level. This prevents fuzzy thought. The pyramidal input gate can be seen in EEG repetition rates at 15 to 40 per second. (Forty Hz). This allows the mind to switch between topics without loosing fidelity. |
||
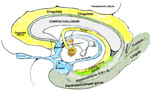
EMOTION AWARENESS EMOTION AND AWARENESSOTIONS AND AWARENESS The capture of emotions by the MIND occurs via connections of sensory memories with the emotion-cortex (cingulate gyrus). In this process, sensory memories of emotion are linked to coincident sensory events. These sensory inputs occur as cascades that temporally bind emotions to pyramidal cell circuitry in sensory (postcentral gyrus ) cortices and through connections to emotional cortices (cingulate gyrus). Emotions are stored in the cingulate gyrus forming the neocortical mass capping the corpus callosum. There are two emotion related cortical regions, 24 and 23 that compose the rostral versus the caudal cingulate. Area 25 is in the septal region, just under the rostrum of the corpus callosum. This site appears to be sensitive to emotional stimulation because the fornix projects through the anterior group nuclei carrying emotions gated memories to the cingulate gyrus through this subcallosal cingulate gyrus path by wrapping anteriorly around the corpus callosum via the cingulate fasciculus. Underlying the cingulate cortex is a large fiber bundle, the cingulate fasciculus. This connection extends from the septal region to the splenium and then ventral through the isthmus into the parahippocampal fasciculus of the hippocampus. This isthmus pathway appears to be a unique reiterative path to refresh emotional states of MIND through visual and auditory inputs. Notably, the essential pathway for remembering is through the hippocampus (via the fornix) to the anterior group nuclei of the dorsal thalamus for temporally gating of emotional events into the cingulate gyrus. Conversion of sensory input to memory is enhanced when emotional events are associated with memories in both time and space locations. The output of the limbic system control of emotions is through the hippocampus that outputs to the dorsal thalamus via the anterior group nuclei. This gates emotion into the emotion cortex, the cingulate gyrus. |
||
COGNITION- SPATIAL/TEMPORAL UNDERSTANDING Out of consciousness comes understanding (cognition) related to time and space. Ordering of events, places, and functional processes is the basis of cognition. This processing occurs in the prefrontal cortex and requires the highest level of processing. Cascading of inputs from conscious sensory memory is essential for the cognitive domain. The medial dorsal, (MD) nucleus dorsal thalamus gates sensory information into the cognitive cortex. Day dreaming is a good example of MIND attention to cognitive processes. The determination of structural and procedural order in understanding arises from MIND’s ability to recall memories and sort them into temporal sequences and spatial order. During thinking, sensory input may be blocked but recalled memories are passed directly to the thalamic gating process for cognition. Memory and emotion form cascades to the cognitive cortex via the medial-dorsal thalamus. The role of the prefrontal cortex is to integrate sensory information into temporal and spatial order. These cascades cycle back into memory stores. Cascades yield a base for the power to reason, to predict, formulate understanding and to make decisions. DECISION-MAKING & SPEECH CONTENT Besides the storing and recall of sensory memories,the highest behavior of humans is assembling speech content and decision-making. The MIND associates sensory inputs with emotions occurring coincident with memory events. Sensory images of facial expressions, body postures, site locations as well as sound are stored as templates in the hippocampus. These are used in comparisons to incoming facial images, places and sounds in order to associate emotion to understanding and decision-making. Visual and auditory cortices connect directly to the hippocampus through the occipital-temporal fasciculus (inferior longitudinal fasciculus) linking the visual cortex to the medial temporal lobe just under the parahippocampal gyrus. This input projects to the hippocampus as the perforant path from the entorhinal cortex. |
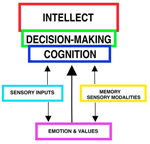 | |
SOURCE OF THE MIND Careful analysis of EEGs in comparison to MEG’s, functional MRI’s, Event–Related-Potentials (ERP’s), local electrode recording and structural-functional organization indicates there are at least four paired thalamo-cortical regions that share information through cascade processing. These functional cortical regions represent MIND's target for inputs: 1) to sensory regions inducing consciousness, 2) memory formation in association with emotions, 3) generation of frameworks of understanding representing cognition (prefrontal cotex), and 4) decision-making as the basis of volitional initiation of behaviors and determination of speech content. Brain waves are evoked field potentials at the surface of the scull. These EEG signals represent cortical activity of the dorsal thalamus that is passing inputs to the cerebral cortex. The waveforms are simplest during non-rapid eye movement in sleep showing a large marker potential designating the beginning of each repeating EEG waveform that can be recorded over each cortical region that is known to receive one of the sensory input types. Analysis of 30 - 40 Hz EEG’s show repeating waveforms occurring every 26-28 milliseconds. In contrast, non-REM sleep or those from unconscious persons show that signals can be as low as 0.5 to 4 per second. Upon awaking, rates range from 5 to 16 Hz as alpha waves having 70 -300 msec interval durations. When memories are recalled at 40 times a second, the CONSCIOUS MIND becomes the COGNIZANT-MIND. The data train follows for 30 msec or less. Thus, the temporal relationships between cascades of MIND functions indicates that thoughts occur over a period of 300 – 600 msec and that MIND actions require only a second or less. Markers in the EEG rates are clear in delta, theta and alpha waves but are difficult to decipher for beta waves because of the short duration of 20 to 30 msec. The thalamic relay of input data into pyramidal cells lasts for only 20 to 30 msec during 40 Hz cognitive events. Thus, all pyramidal cells are being normalized to the same resting potentials at the beginning of the thalamic relay-barrage. (See Google/brainwaves). The origin of the synchronizing signal, I propose, is from intralaminar thalamic neurons projecting to the surface layer (Layer I) of the neocortex. I believe the large marker potential of the EEG ‘s represents massive pyramidal cell depolarization producing synchronous depolarization of all pyramidal cells by voltage-gated calcium channels projecting to layer one dendrites of pyramidal cells. Here, the intralaminar thalamic axons can synapse on all pyramidal cells because all apical dendrites end in the sub-pial layer. The voltage-gated calcium channels in these dendrites could produce massive depolarizations of all pyramidal cells that are seen as the EEG marker preceding data inputs. I suggest that the marker-signal resets the resting potentials of all pyramidal cells by depolarization of the cytosol. This is followed directly by repolarization of all pyramidal cells at the same time. The result is equated resting potentials across the cortex at the time pyramidal cells begin to receive sensory inputs from the dorsal thalamus relay. The result is that synaptic inputs are integrated aross the cortex with maximal efficacy over the next 20 to 30 msec. Pyramidal cell potential resetting is essential in order for the thalamus to gate synaptic input packets of 5 or less synaptic events during each ERP or EEG cycle (a synaptic event lasts only 3 msec). Synchronous thalamic gating to pyramidal cells ensures reception fidelity for handshaking of signals within and across four cortical regions. Furthermore, this allows a rapid shifting of MIND focus between subjects without loss of fidelity. Timing in circuitry between thalamic gated components is of highest importance in order to have cascade processing of sensory inputs and memory recall into cognition and onto decision making and speech generation. For example, sensory input forms the basic information for emotion and cognition and must arrive to the decision making target in sequence. Cascading allows sensory input processing that is followed by emotion assessment and then cognitive evaluation providing fodder for behavioral decision making. |
|
|